Although useful to explain the reactivity and chemical bonding of certain elements the Bohr model of the atom does not accurately reflect how electrons are spatially distributed surrounding the nucleus. In which Ap represents the uncertainty in momentum of the electron and.
BK9780854046478-00001pdf - Read File Online - Report Abuse.

. The correct answer was given. Example of an element that has an electron distribution ending in. Electrons have a specific form of distribution or configuration in every atom even Barium.
Nevertheless check the complete configuration and other interesting facts about Barium that most people. For example the electron configuration of a neon atom is 1s 2 2s 2 2p 6. The subshells have a distinct shape and configuration in which the electrons move freely.
The transition metals are behind by one period because the d electrons are high in energy. The electron configuration states where electrons are likely to be in an atom. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the elements symbol.
July 5th 2013 100155 AM. Some are hard to memorise or predict so what is the electron configuration of an atom of Ba. The complete electron configuration of Ca is 1s22s22p63s23p64s2.
Some are hard to memorise or predict so what is the electron configuration of an atom of Sc. As we learned earlier each neutral atom has a number of electrons equal to its number of protons. Electron configuration was first conceived under the Bohr model of the atom and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.
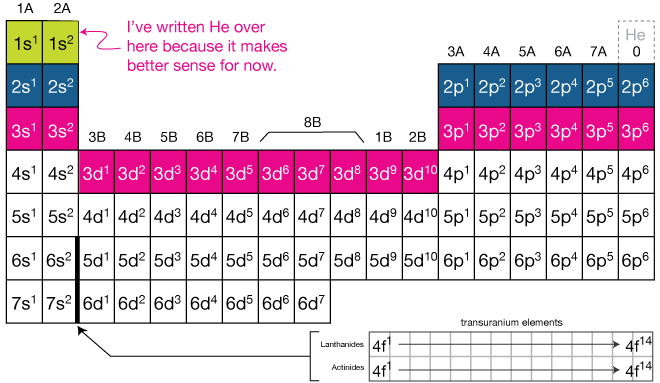
Electron configurations are the summary of where the electrons are around a nucleus. For the rare earth elements the Lanthanides and Actinides they end in f. In order to write the Na electron configuration we first need to know the number of electrons for the Na atom there are 11 electrons.
The complete electron configuration of Na 1s22s22p63s1. Electrons have a specific form of distribution or configuration in every atom even Scandium. Example of an element that has an electron distribution ending in s2d2 is Ca or calcium.
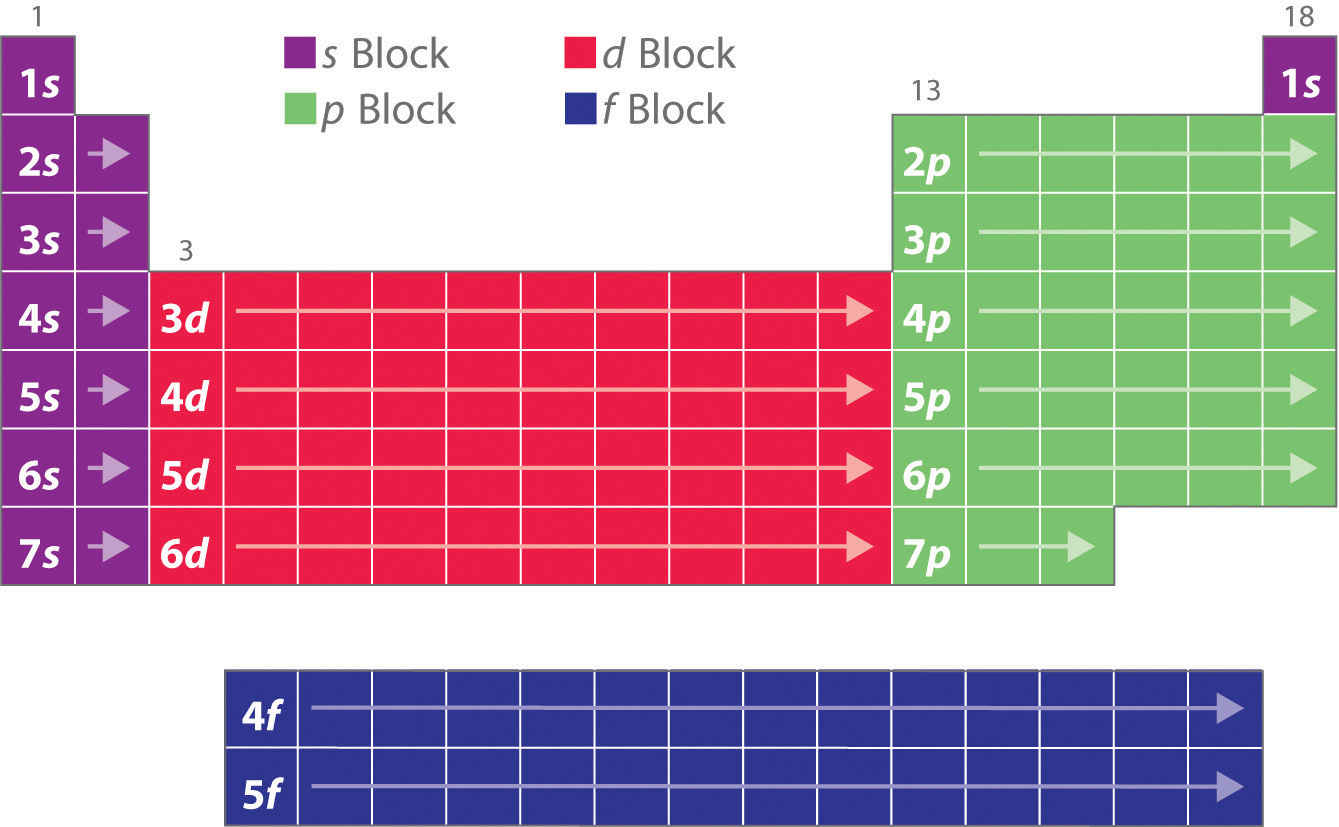
The general rule is that the elements electron configuration ends in d and whatever place they are in. An electron configuration can quickly and simply tell a reader how many electron orbitals an atom has as well as the number of electrons populating each of. Electron orbitals are differently-shaped regions around an atoms nucleus where electrons are mathematically likely to be located.
Scandium would end in 3d1 titanium in 3d2 etc. To explain Chromiums electron configuration we could introduce. Since 1s can only hold two electrons the next 2.
Here is a summary of the. All the electron attributes are exact and could be and are used as a standard for measurement. Which element has the electron distribution s2d2.
Use the element blocks of the periodic table to find the highest electron orbital. How to Find Electron Configuration. Example of an element that has an electron distribution ending in s2p1 is Na or sodium.
But are based upon the distribution of v Val-. Ending with the incom-Filename. Each shell and subshell have a limitation on the amount of electrons that it can carry.
Of the characteristics of an electron is that it has near zero sizeless that 10-18 m nearly infinite potential at its center possesses gravitational and electrical forces that reach the end of the universe. Alternatively remember group 1 alkali metals and group 2 alkaline earth metals are s-block. If you dont have a chart you can still find the electron configuration.
We start at lithium on the periodic table and we see it is in the second row and the first column of the s-block so its electron configuration ends in. Explain Bohr Bury rules for Distribution of Electrons into Different Shells Electron distribution The distribution of electrons in different orbits or shells is governed by a scheme known as Bohr bury schemeThe arrangement of electrons in various energy levels of an atom is known as the electronic configuration of the atom. A beryllium atom with two valence electrons would have the electron dot diagram below.
Nevertheless check the complete configuration and other interesting facts about Scandium. Helpful 0 Not Helpful 5 Add a Comment. In writing the electron configuration for sodium the first two electrons will go in the 1s orbital.
It can also be noted that this isotope of hydrogen is radioactive. What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located. Brief Summary Atomic Basis Theory - Royal Society of.
The correlated and uncorrelated distributions are very similar. The misorientation angle distribution. This isotope of hydrogen contains 1 proton 1 electron and 1 neutron.
The maximum electrons that can be carried by the sub-shell S is 2 by P is 6 by D is 10 and the F sub-shell can carry 14. In the case of Barium the abbreviated electron configuration is Xe 6s2. This isotope of hydrogen contains 1 proton 1 electron and no neutrons.
This isotope of hydrogen contains 1 proton 1 electron and 2 neutrons. Electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals. When we write the configuration well put all 11 electrons in orbitals around the nucleus of the Sodium atom.
The distribution for CPTi has a greater proportion of misorientations in the 2050 range and conversely a smaller proportion in the 5090 range than the theoretical curve ending up with the effect that the distribution is skewed towards medium misorientation angles. These relatively complex shapes result from the fact that electrons behave. The K shell contains a 1s subshell.
They do not circle the nucleus like the earth orbits the sun but are rather found in electron orbitals. The complete electron configuration of Na 1s22s22p63s1. The complete electron configuration of Na 1s22s22p63s 1.
Example of an element that has an electron distribution ending in s2p1 is Na or sodium. This decides the electron capacity of the shells. An electron shell is the set of allowed states that share the same principal quantum number n the number before the letter in the orbital label that electrons.
The exchange energy Pi_e a stabilizing quantum mechanical factor that is directly proportional to the number of pairs of electrons in the same subshell or very close-energy subshells with parallel spins The coulombic repulsion energy Pi_c a destabilizing factor that is inversely proportional to the. Example of an element that has an electron distribution ending in s2p1 is Na or sodium. The charge mass spin and magnetic dipole moment are of them.
In the case of Scandium the abbreviated electron configuration is Ar 3d1 4s2. An atoms electron configuration is a numeric representation of its electron orbitals. Since electrons repel each other the dots for a given atom are distributed evenly around the symbol before they are paired.
Electron Distribution Ending In S2 D2.
6 9 Electron Configurations And The Periodic Table Chemistry Libretexts
0 Comments